how to find core electrons|3.4: Core and Valence Electrons : Tagatay Learn how to determine the electron configuration of atoms using the Aufbau principle, Pauli exclusion principle, and Hund's rule. This web page is part of a free textbook on . This is a list of national capitals, including capitals of territories and dependencies, non-sovereign states including associated states and entities whose sovereignty is disputed.. The capitals included on this list are those associated with states or territories listed by the international standard ISO 3166-1, or that are included in the list of states with limited .Schools offering Accountancy / Accounting courses in Isabela A list of universities and colleges offering Accountancy / Accounting courses in Isabela, Philippines. Whenever possible we provide full details about the courses in each of the schools, including tuition fees, admission requirements, course description and the admission phone number. .
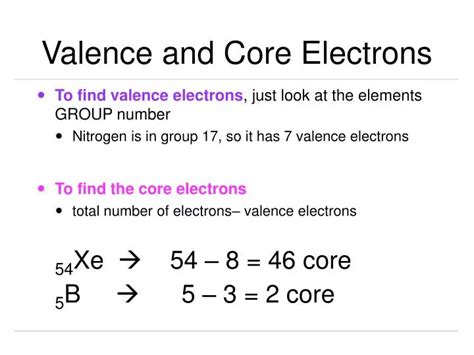
how to find core electrons,The electrons of an atom are typically divided into two categories: valence and core electrons. Valence electrons occupy the outermost shell or highest energy . Learning Objective: Evaluate the number of valence electrons and core electrons from the electron configurations.Topics: core electrons, valence electrons
We can see from the electron configuration of a carbon atom—1s 2 2s 2 2p 2 —that it has 4 valence electrons (2s 2 2p 2) and 2 core electrons (1s 2). You will see in .Learn how to determine the electron configuration of atoms using the Aufbau principle, Pauli exclusion principle, and Hund's rule. This web page is part of a free textbook on .Core electrons are the electrons in an atom that are not valence electrons and do not participate in chemical bonding. The nucleus and the core electrons of an atom form the .Since the core electron shells correspond to noble gas electron configurations, we can abbreviate electron configurations by writing the noble gas that matches the core electron configuration, along with the .

Learn how to identify valence electrons, the electrons in the outermost shell of an atom, and how they participate in chemical reactions. See examples, definitions, and questions from other learners in the comments section. How to Find Core Electrons? To find the number of core electrons in an atom, you can use the periodic table to identify the element and then subtract the . Since germanium is a main-group element, its valence electrons are those in the outermost principal energy level. For germanium, the n = 1, 2, and 3 principal levels are complete (or full) and.Core electrons are the electrons in an atom that are not valence electrons and do not participate in chemical bonding. The nucleus and the core electrons of an atom form the atomic core. Core electrons are tightly bound to the nucleus. Therefore, unlike valence electrons, core electrons play a secondary role in chemical bonding and reactions by . Valence electrons. Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s .Electron Shielding and Effective Nuclear Charge. If an electron is far from the nucleus (i.e., if the distance \(r\) between the nucleus and the electron is large), then at any given moment, many of the other electrons will be between that electron and the nucleus (Figure \(\PageIndex{1}\)). Hence the electrons will cancel a portion of the positive charge of the .
Assigning Electron Configuration . We write electronic configurations by following the aufbau principle (from German, meaning “building up”). First we determine the number of electrons in the atom; then we add electrons one at a time to the lowest-energy orbital available without violating the Pauli Exclusion Principle .That is, recognizing that each .
The electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying the inner shell orbitals are called core electrons (Figure 6.28). Since the core electron shells correspond to noble gas electron configurations, we can abbreviate electron configurations by writing the noble gas that . Core electrons are the electrons that are found in the innermost electron shell of an atom. Core electrons are sometimes also referred to as “inner shell electrons.”. Core electrons are tightly bound to the nucleus and have the highest electron binding energy. The number of core electrons in an atom depends on the atomic number of the .how to find core electrons 3.4: Core and Valence Electrons Figure 8.4.1 8.4. 1: (a) The radius of an atom is defined as one-half the distance between the nuclei in a molecule consisting of two identical atoms joined by a covalent bond. The atomic radius for the halogens increases down the group as n increases. (b) Covalent radii of the elements are shown to scale. 2. Subtract the charge from the atomic number if the ion is positive. If the charge is positive, the ion has lost electrons. To determine how many electrons are left, subtract the amount of charge from the atomic number. In this case, there are more protons than electrons.
The atomic core is made up of an atom’s nucleus and core electrons. The nucleus is tightly connected to the core electrons. What number of core electrons does MG have? Mg has 10 core electrons and two valence electrons as a result of the 10 core electrons. The number of core electrons is determined by the element’s period (row). (equal to . Core electrons don’t bond because they, like noble gases, are stable, feeling the greatest amount of charge from the atomic nucleus. However, valence electrons feel an effective charge from the nucleus, or a charge brought about after the positive charge of the nucleus is subtracted by the number of core electrons. This effective charge is . The third major category of elements arises when the distinguishing electron occupies an f subshell. The first example occurs in the case of the lanthanoids (elements having atomic numbers between 57 and 71).The lanthanoids have the general electron configuration [Kr]4d 10 4f i 5s 2 5p 6 5d 0 or 1 6s 2. where i is a number .
In Figure 1B, if a 2p electron exists at a distance r 1, most likely the 1s electrons (core electrons) will be between the electron of interest and the nucleus. But, there is only a small probability of the 2 s electron .

This video explains the difference between the three types of electrons and demonstrates it in an example.Support us!: https://www.patreon.com/learningsimply.Note that while we often refer to the Z eff of a valence electron, we can calculate the Z eff for any electron by taking into account only the number of core electrons that are shielding. For example, consider a 2 s electron of Cl. For Cl, Z = 17 and the electron configuration is 1 s2 2 s2 2 p6 3 s2 3 p5. The only electrons that will shield a 2 . When electrons are removed in succession from an element, the transition from removing valence electrons to removing core electrons results in a large jump in ionization energy. By . In this video, I explain the following student's question "How many inner, outer, and valence electrons are present in an atom of Manganese?" I break it down. So an electron being added to the third shell (the 3s orbital) would feel no attraction to the nucleus and not remain easily bound to the neon atom. But if we do the same to a neutral atom . First, use the periodic table and see the number of electrons for a neon atom is 10. Follow the Aufbau principle and fill electron shells: 1s 2 2s 2 2p 6; Write the noble gas configuration using the noble gas core before neon on the periodic table, followed by the valence electrons. The noble gas configuration of neon is [He] 2s 2 2p 6. Notice .
how to find core electrons|3.4: Core and Valence Electrons
PH0 · ⚗️ Identify Valence Electrons and Core Electrons
PH1 · What are Core Electrons?
PH2 · Valence electrons (video)
PH3 · How to calculate core electrons
PH4 · How do you find core and valence electrons? + Example
PH5 · Core electron
PH6 · CHEMISTRY 101: Valence and core electrons
PH7 · 6.4 Electronic Structure of Atoms (Electron Configurations)
PH8 · 3.4: Core and Valence Electrons
PH9 · 3.1: Electron Configurations
PH10 · 1.9B: Valence and Core Electrons